
Nuveen California Select Tax-Free Income Portfolio (NXC) News
Market Cap: $79.72M
Avg Volume: 21.38K
Industry: Asset Management - Income
Sector: Financial Services

Sell Equities, Buy Municipal Debt At A 7.21% Discount With Nuveen California Select Tax-Free Income Portfolio
Municipal CEFs like Nuveen California Select Tax-Free Income Portfolio offer tax-free returns, low leverage, and are currently trading at a discount. NXC invests in high-quality municipal securities, with an average coupon of 3.68% and a distribution rate on NAV of 3.97%. The fund's market price is $13.17, NAV is $13.69, and it's trading at a 3.80% discount, presenting a potential profit opportunity.
seekingalpha.com
Read More
Immix Biopharma Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for NXC-201, sterically-optimized CAR-T for relapsed/refractory AL Amyloidosis
FDA RMAT designation follows positive proof-of-concept U.S. clinical data from the NXC-201 NEXICART-2 clinical trial in relapsed/refractory AL Amyloidosis RMAT designation potentially streamlines the path to market approval by allowing frequent interactions with FDA and routes to FDA Accelerated Approval and Priority Review Enrollment in NEXICART-2 study accelerating; next update planned for H1 2025 LOS ANGELES,CA, Feb. 10, 2025 (GLOBE NEWSWIRE) -- Immix Biopharma, Inc. (Nasdaq: IMMX) (“ImmixBio”, “Company”, “We” or “Us”, “IMMX”), a clinical-stage biopharmaceutical company developing cell therapies for AL amyloidosis and select immune-mediated diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted RMAT designation to sterically-optimized CAR-T NXC-201 for the treatment of relapsed/refractory AL amyloidosis. As of June 2024 public information, FDA approved less than half of RMAT applications submitted to the agency during the last eight years.
globenewswire.com
Read More
Immix Biopharma Accelerates Enrollment in U.S. AL Amyloidosis Trial of NXC-201 CAR-T
LOS ANGELES, Jan. 07, 2025 (GLOBE NEWSWIRE) -- Immix Biopharma, Inc. (Nasdaq: IMMX) (“ImmixBio”, “Company”, “We” or “Us”, “IMMX”), a clinical-stage biopharmaceutical company developing cell therapies for AL Amyloidosis and select immune-mediated diseases, today announced successful completion of the six-patient Phase 1b safety run-in segment in the U.S. NEXICART-2 study of NXC-201, an investigational CAR-T therapy, in patients relapsed/refractory (R/R) AL Amyloidosis. Achievement of this milestone is expected to accelerate enrollment across U.S. study sites beginning in January 2025.
globenewswire.com
Read More
Journal of Clinical Oncology Publishes NXC-201 Positive Clinical Results in relapsed/refractory AL Amyloidosis
Chimeric antigen receptor T-cell (CAR-T) cell therapy NXC-201 is a novel approach to treat relapsed/refractory AL Amyloidosis NXC-201 demonstrated compelling clinical activity, rapid and deep complete responses in frail and resistant relapsed/refractory AL Amyloidosis patients LOS ANGELES, Dec. 16, 2024 (GLOBE NEWSWIRE) -- Immix Biopharma, Inc. (“ImmixBio”, “Company”, “We” or “Us” or “IMMX”), a clinical-stage biopharmaceutical company developing cell therapies for AL Amyloidosis and select immune-mediated diseases, announced today the Journal of Clinical Oncology (JCO) published NXC-201 clinical results in relapsed/refractory AL Amyloidosis. The data reported on 16 enrolled patients in NEXICART-1 who had received a median 4 prior lines of therapy prior to treatment with NXC-201.
globenewswire.com
Read More
Nuveen Closed-End Funds Declare Distributions and Updates to Distribution Policies
NEW YORK--(BUSINESS WIRE)--Nuveen Closed-End Funds today announced that the Board of Trustees of the Funds has approved the regular monthly and quarterly distributions. In addition, the Board of Trustees has approved updated distribution polices described below under “Monthly & Quarterly Distributions” for The Nuveen Preferred & Income Opportunities Fund (NYSE: JPC), Nuveen Preferred and Income Term Fund (NYSE: JPI), Nuveen Variable Rate Preferred & Income Fund (NYSE: NPFD), Nuveen.
businesswire.com
Read More
Weekly Closed-End Fund Roundup: January 8, 2023
21 out of 22 CEF sectors were positive on price and 20 out of 22 sectors were positive on NAV last week. MLPs are the most discounted CEF sector.
seekingalpha.com
Read More
Weekly Closed-End Fund Roundup: VFL Tender Results (December 18, 2022)
2 out of 23 CEF sectors were positive on price and 2 out of 23 sectors were positive on NAV last week. VFL offers tender results.
seekingalpha.com
Read More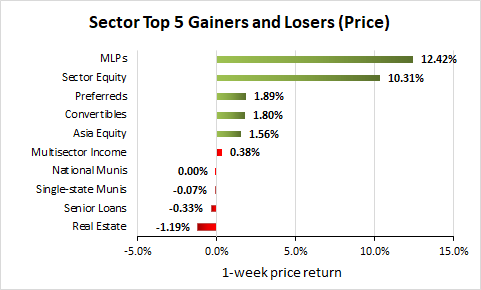
Weekly Closed-End Fund Roundup: June 21, 2020
4 out of 23 CEF sectors positive on price and 6 out of 23 sectors positive on NAV last week. MLPs lead while commodities lag. Preferreds have the highest sector
seekingalpha.com
Read More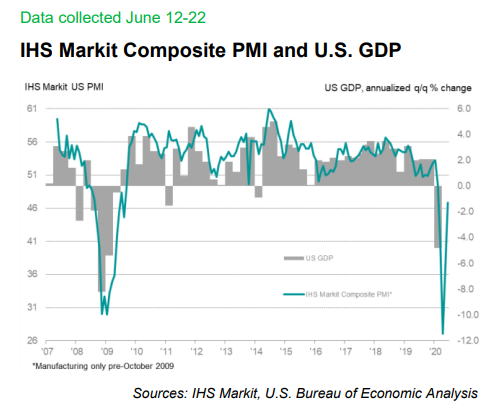
The Reopening Killed The V-Shaped Recovery
This is a weekly series focused on analyzing the previous week’s economic data releases. The objective is to concentrate on leading indicators of economic activ
seekingalpha.com
Read More